
During my early years in the natural health field, I had a lot of unanswered questions and confusion about the whole subject of fats. First of all, the general attitude was that too much of any kind of fat was bad, but then there seemed to be certain kinds of fats that were good for us. So I did what I always do when I don't understand something, I did some research on it. I hope this article will help you with your own research into understanding fats better so you can select healthy fats and avoid unhealthy fats.
Understanding Triglycerides
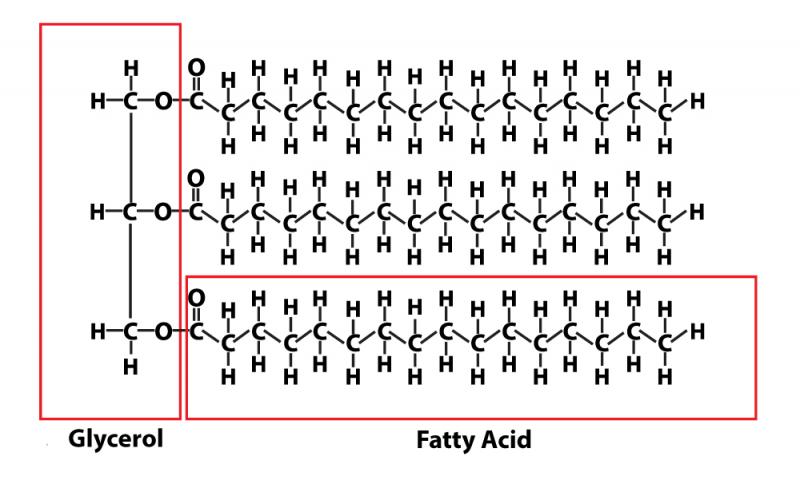 The fats and oils we find in foods are composed of triglycerides. You've probably heard someone talk about having a high level of triglycerides on a blood test; that simply means they have high levels of fat in their blood.
The fats and oils we find in foods are composed of triglycerides. You've probably heard someone talk about having a high level of triglycerides on a blood test; that simply means they have high levels of fat in their blood.
Triglycerides are composed of three fatty acids attached to one molecule of glycerine as shown on the right. When fats are digested, the bonds the fatty acids have with the glycerine are broken. That's the job of the lipase (fat-digesting) enzymes from the pancreas. The bile secreted from the liver has the purpose of making these fats water soluble so they can be absorbed and transported through the bloodstream.
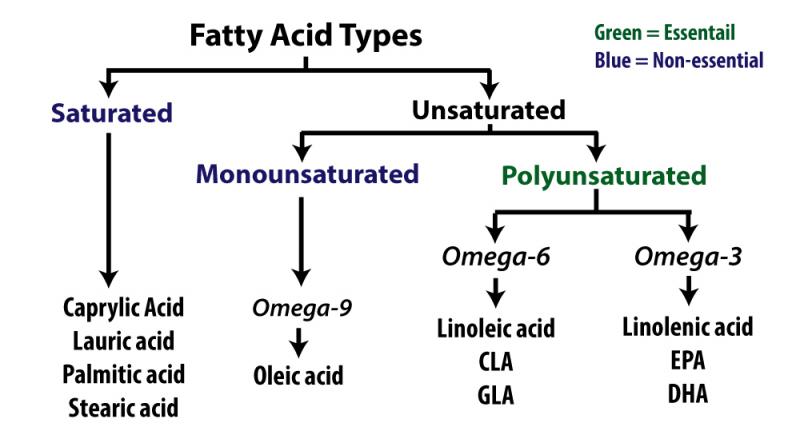 The properties of various fats and oils (triglycerides) are determined by the type of fatty acids they contain. Fatty acids come in many varieties. They may be saturated or unsaturated. If they’re unsaturated they can be mono- or poly-unsaturated and the polyunsaturated fatty acids can be either the omega-3 or omega-6 type as shown in the chart on the left. They may also be long, medium, or short-chained. In the first part of this article, we'll explain what all of this means.
The properties of various fats and oils (triglycerides) are determined by the type of fatty acids they contain. Fatty acids come in many varieties. They may be saturated or unsaturated. If they’re unsaturated they can be mono- or poly-unsaturated and the polyunsaturated fatty acids can be either the omega-3 or omega-6 type as shown in the chart on the left. They may also be long, medium, or short-chained. In the first part of this article, we'll explain what all of this means.
Saturated versus Unsaturated Fatty Acids
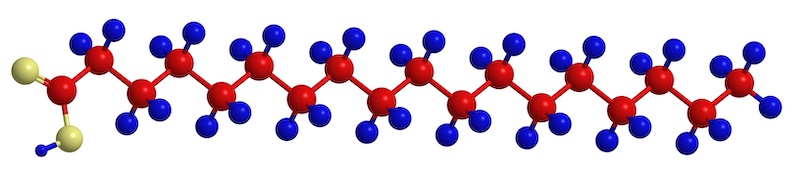 The fatty acids themselves are composed of long chains of carbon molecules that are bonded to hydrogen atoms. Something that helps me understand all this chemistry stuff is to think of various molecules as having differing numbers of hands which they can use to hold onto other molecules. Hydrogen has one hand. Oxygen has two hands. Carbon has four. So, each carbon atom can hold hands with the carbon in front of it and the carbon behind it. This leaves two free hands to hold onto two hydrogen atoms.
The fatty acids themselves are composed of long chains of carbon molecules that are bonded to hydrogen atoms. Something that helps me understand all this chemistry stuff is to think of various molecules as having differing numbers of hands which they can use to hold onto other molecules. Hydrogen has one hand. Oxygen has two hands. Carbon has four. So, each carbon atom can hold hands with the carbon in front of it and the carbon behind it. This leaves two free hands to hold onto two hydrogen atoms.
In the illustration above, the carbon atoms are red and the hydrogen atoms are blue. (The yellow ones are oxygen atoms that bond at one end of the chain.) You can see that each carbon atom is bonded to two hydrogen atoms, except for the final one, which is attached to three. The hydrogen atoms stick out sort of like Mickey Mouse ears. Because all of the carbons in the chain (not at the ends) are holding onto two hydrogen atoms the fatty acid pictured above is saturated.
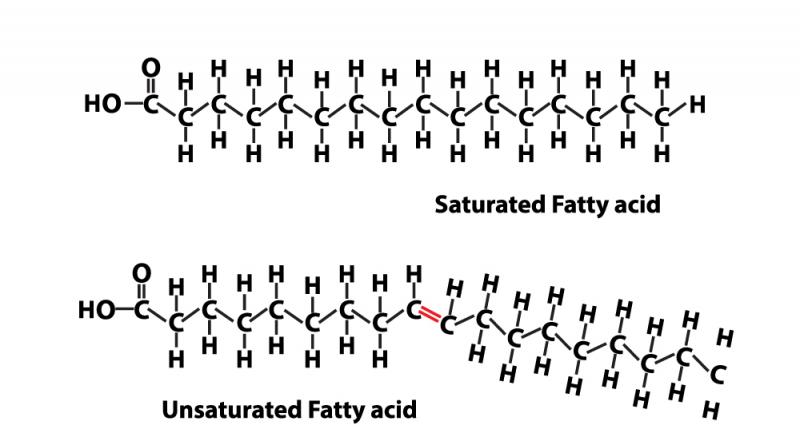 If one or more pairs of carbon atoms are missing hydrogens, the fatty acid would be unsaturated. When the two carbons don't have a hydrogen atom to hold onto, they hold hands with each other, instead. This makes them double-bonded. In the illustration on the left, the double bond is highlighted in red.
If one or more pairs of carbon atoms are missing hydrogens, the fatty acid would be unsaturated. When the two carbons don't have a hydrogen atom to hold onto, they hold hands with each other, instead. This makes them double-bonded. In the illustration on the left, the double bond is highlighted in red.
Saturation affects the structure of the fatty acid. Saturated fatty acids are straight, while most unsaturated fats bend at the points where a carbon pair is missing hydrogen atoms. The bend makes the fat more fluid, which is why polyunsaturated fats tend to be liquid at room temperature, whereas saturated fats tend to be solid at room temperature.
This affects how they act in the body. Polyunsaturated fats help keep cell membranes pliable, while saturated fats make them stiffer. This influences the permeability of the membrane too. Too many saturated fats contribute to problems like insulin resistance.
The Omega Factor
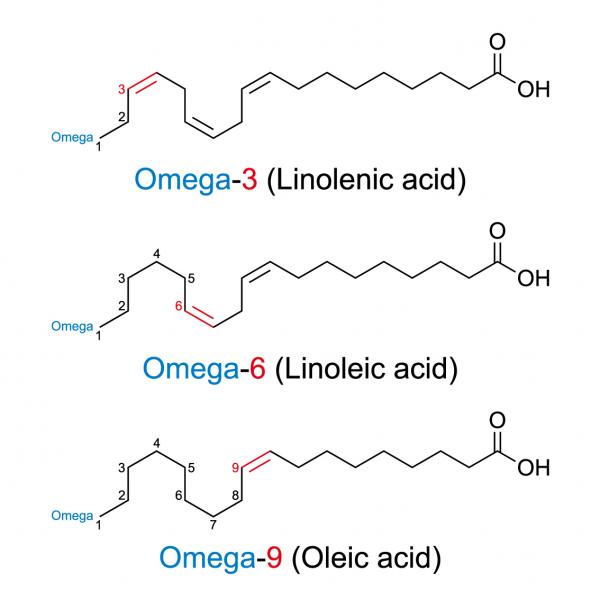 Unsaturated fatty acids fall into three basic categories: omega-3, omega-6, and omega-9. The number following the omega is derived by counting the position of the first set of double bonds where hydrogen molecules are missing. This is illustrated on the right. The first double bond in an omega-3 fatty acid is in the third pair, while it's in the sixth pair in an omega-6 fatty acid.
Unsaturated fatty acids fall into three basic categories: omega-3, omega-6, and omega-9. The number following the omega is derived by counting the position of the first set of double bonds where hydrogen molecules are missing. This is illustrated on the right. The first double bond in an omega-3 fatty acid is in the third pair, while it's in the sixth pair in an omega-6 fatty acid.
Omega-3 and omega-6 fatty acids are known as essential fatty acids (EFA). This is because the body can't make them. They have to be derived from the diet. The basic omega-3 EFA is linolenic acid and the basic omega-6 EFA is linoleic acid. The body uses these to make longer-chain fatty acids such as EPA and DHA but it can't make the basic form.
Omega-9 fatty acids, which occur when the double-bond is in the ninth pair, aren't essential. Oleic acid is the basic omega-9 essential fatty acid and is found in high amounts in olive oil and avocados. It is considered a healthy fatty acid because it lowers the risk of heart disease.
The ratio of omega-6 to omega-3 EFAs is also important. It's easy to obtain adequate amounts of omega-6 in modern society because vegetable oils are generally high in omega-6 EFAs. Most people are not getting enough omega-3 EFAs, which is a problem.
Oils are Mixtures of Fatty Acids
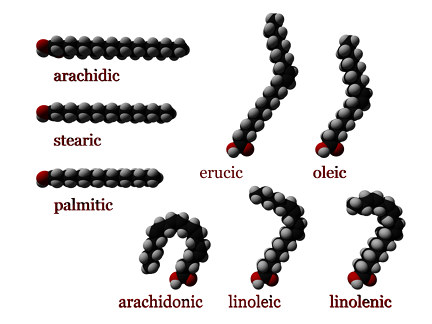 The picture on the left shows three saturated fatty acids (arachidic, stearic, and palmitic) and two monounsaturated fatty acids (erucic and oleic). It also shows three polyunsaturated fatty acids (arachidonic, linoleic, and linolenic).
The picture on the left shows three saturated fatty acids (arachidic, stearic, and palmitic) and two monounsaturated fatty acids (erucic and oleic). It also shows three polyunsaturated fatty acids (arachidonic, linoleic, and linolenic).
Fats are a mixture of all three types of fatty acids, in varying proportions. For example, coconut oil is 92% saturated, 6% monounsaturated, and 2% polyunsaturated fatty acids. Olive oil, on the other hand, is considered a monounsaturated fat because it is 15% saturated, 73% monounsaturated, and 12% polyunsaturated fatty acids. Safflower oil is a polyunsaturated fat because it is 10% saturated, 13% monounsaturated, and 77% polyunsaturated fatty acids.
The more saturated fatty acids in an oil the more likely it will be solid at room temperature. The more unsaturated fatty acids, the more liquid it will be.
It's also not correct to say that saturated fats are bad and unsaturated fats are good. It's not that simple. The length of the fatty acid also makes a difference. So, let's talk about that next.
Fatty Acid Length
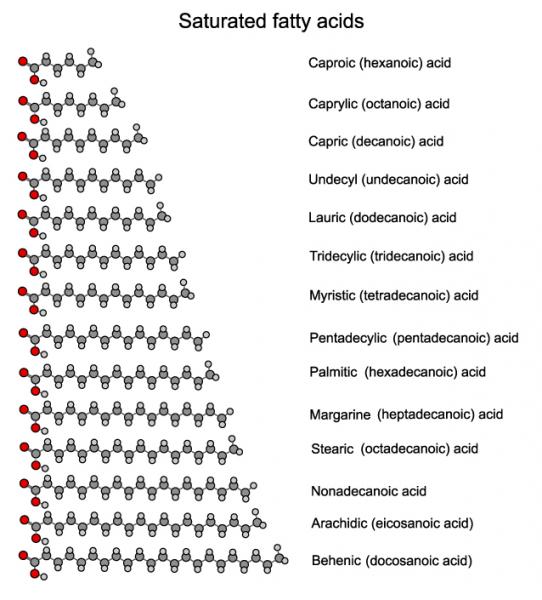
To the right is a graphic showing the length of various saturated fatty acids. Fatty acids are categorized as short, medium, and long-chained. The short-chain fatty acids have less than six carbon pairs. The longer chained ones have eleven or more. In between are the medium-chained fatty acids.
Oils high in medium-chained fatty acids, known as medium chain triglycerides or MCTs for short have been discovered to have many health benefits. So have short-chained fatty acids. This is why it's overly simplistic to categorize all saturated fats as bad. Coconut oil, palm oil, butter, and even lard have a lot of medium and short-chained saturated fatty acids and can be healthy.
The real source of bad fats is the modern refined vegetable oils and deep-fried foods. So, let's talk about those.
Unsaturated Oils and Transfats
There's a problem with unsaturated fats. The double bonds are sites on the molecule where the fat can be oxidized. In other words, a molecule of oxygen can attach where the double bond is located in place of two hydrogen atoms. Remember that oxygen has two hands. This makes the oil rancid, which not only gives it an off taste, it also ruins the oil nutritionally. There is evidence that it is oxidized or rancid fats that stick to your arteries and contribute to cardiovascular disease.
There is also a problem when these oils are heated. Polyunsaturated fats form something called trans fats when used for cooking. The higher the heat and the longer they are used, the more trans fats they will form.
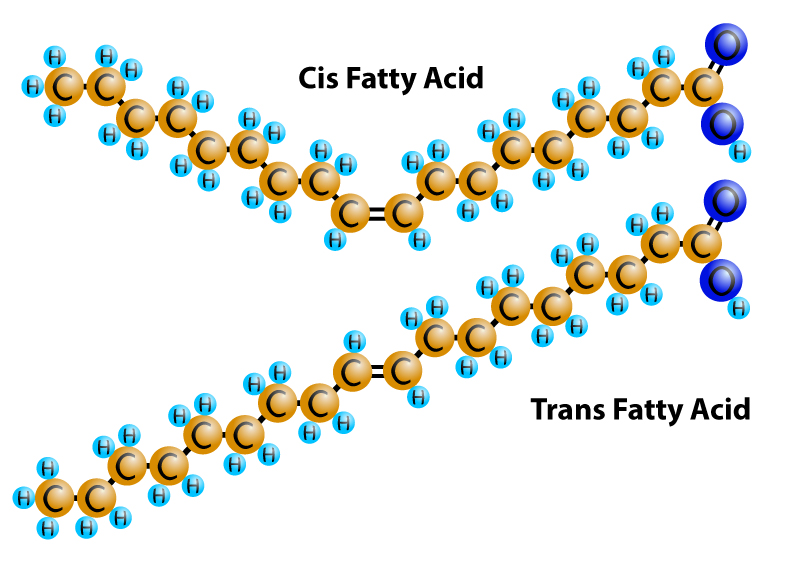 To understand trans fats, we need to look again at fatty acid structure. Normally, in an unsaturated fatty acid, the hydrogen molecules in a double bond are attached on the same side. This is known as a cis-fatty acid. It's cis-fatty acids that bend as shown in the illustration on the left. In a transfat, the hydrogen molecules attach to opposite sides of the double bond. This keeps the fatty acid straight so it acts more like a saturated fat than an unsaturated fat.
To understand trans fats, we need to look again at fatty acid structure. Normally, in an unsaturated fatty acid, the hydrogen molecules in a double bond are attached on the same side. This is known as a cis-fatty acid. It's cis-fatty acids that bend as shown in the illustration on the left. In a transfat, the hydrogen molecules attach to opposite sides of the double bond. This keeps the fatty acid straight so it acts more like a saturated fat than an unsaturated fat.
There's a lot of research suggesting transfats are unhealthy. First, like oxidized fats, they are also associated with arteriosclerosis and an increased risk of heart disease. They also increase insulin resistance and contribute to diabetes, something they have in common with long-chain saturated fats. They may also increase inflammation and cancer risk. The biggest source of these transfats is processed foods and foods that have been deep-fried in vegetable oils that have been repeatedly reheated.
Refining and Hydrogenating Oils
 To increase shelf life, vegetable oils are refined and may also be partially or completely hydrogenated. Let's look at both of these processes.
To increase shelf life, vegetable oils are refined and may also be partially or completely hydrogenated. Let's look at both of these processes.
Fresh oils pressed from nuts and seeds contain vitamins, minerals, and other nutrients. Because these other nutrients decrease shelf life, they are removed from oils, just like nutrients are removed from sugar and white flour. Plus, the extraction process itself can involve heat which causes transfats to form.
Processed oils are de-gummed to remove phospholipids like lecithin and minerals like iron, copper, calcium, and magnesium. Sodium hydroxide may added to remove free fatty acids (fatty acids which are not attached to glycerine molecules). This removes more minerals and phospholipids. Oils are then bleached and deodorized to remove beta-carotene, aromatic oils, (which give natural oils flavor and odor), and any remaining free fatty acids.
Fat-soluble vitamins, which are present in many natural fats may also be removed. Vitamins like vitamin A (both carotenoid and retinol forms), vitamin D, and vitamin E help prevent oils from oxidizing. The lack of these vitamins also contributes to heart disease and other chronic health problems.
Hydrogenation is a process that is used to add hydrogen molecules to some of those carbon bonds. Most commercial cooking oils, and all margarine and shortening products, are composed of partially hydrogenated fats. To say that another way, some of their unsaturated fatty acids have been turned into saturated fats. The problem is that in nature these hydrogen molecules are added in a controlled way, while in the hydrogenation process, they are added randomly. This results in the production of trans fats.
Choosing Healthy Fats
 The best sources of fat are natural foods eaten in their whole-food form. These fats contain the vitamins, minerals, and other nutrients destroyed in the refining process. Good plant sources of fat include avocados, nuts (particularly walnuts, macadamia nuts, and almonds), and seeds (e.g. hemp, flax, and chia). These foods can provide a feeling of fullness that helps you eat less, as well as the fatty acids you need for good health.
The best sources of fat are natural foods eaten in their whole-food form. These fats contain the vitamins, minerals, and other nutrients destroyed in the refining process. Good plant sources of fat include avocados, nuts (particularly walnuts, macadamia nuts, and almonds), and seeds (e.g. hemp, flax, and chia). These foods can provide a feeling of fullness that helps you eat less, as well as the fatty acids you need for good health.
Contrary to popular belief, not all animal fats are bad. The biggest problem with animal fat is its source. The fatty acid profile in animals raised in feed lots is very different from that of animals raised on pasture or obtained from wild sources. Also, many toxins accumulate in fat, so animals that are fed food containing pesticides or are given chemicals to fatten them up or increase production of milk and eggs, will have less healthy fats.
On the other hand, if you can get organic, grass-fed animal products like eggs, butter, cream, whole milk, raw cheese, poultry, or even red meat, enjoy them. Fish can also be a good source of healthy fats, including wild-caught salmon, sardines, tuna, wild-caught trout, and herring.
Selecting the Right Oils
 Never use oils high in polyunsaturated fats like flax, hemp, pumpkin, or walnut oil for cooking. These oils should be used in salads or other dishes that don’t require heat. It’s also good to refrigerate them as they can go rancid more easily.
Never use oils high in polyunsaturated fats like flax, hemp, pumpkin, or walnut oil for cooking. These oils should be used in salads or other dishes that don’t require heat. It’s also good to refrigerate them as they can go rancid more easily.
Heating any oil above 300 degrees Fahrenheit causes alterations in the unsaturated fatty acids. At 320 ºF trans-fatty acids begin to form. The higher the temperature, the more trans fats that are formed. Many fast-food restaurants use the same oil for many days. They simply filter the used oil and top it off with some fresh oil. This means these deep-fried foods can contain a lot of trans fats.
If you need to fry something, oils high in saturated fats are better because oils that are already saturated won't form trans fats. They can also withstand heat better. Oils have a smoke point, which is where they start to break down and form unhealthy compounds. If you cook regularly and you see smoke starting to come off the oil you will know that the oil is too hot and is starting to break down.
Butter and coconut oil have a smoke point of 350 ºF and extra virgin olive oil’s smoke point is 375 ºF. So while these oils can be used for cooking, they aren’t the best for frying. Oils with a higher smoke point include canola, corn, peanut, and soybean oil, which have smoke points been 400 ºF and 450 ºF. However, most of these oils are highly refined and/or partially hydrogenated.
I think the best oils for deep frying are lard and palm oil, followed by avocado oil. The best oils for low-temperature cooking are coconut oil, olive oil, ghee (clarified butter). Other potentially good cooking oils are sesame and sunflower oil. I recommend avoiding soy, canola, cottonseed, corn, and grapeseed oil. I hope all of this has helped you understand fats better and know which ones to use as well as which ones to avoid.
Steven's Articles
-

-
Reishi (Ganoderma) Mushroom
A TCM remedy for calming the shen (spirit), balancing…
-

-
Eucommia Bark
A superior tonic that promotes kidney, structural,…
January
-

-
Goldenthread, Phellodendron, and Yellow Root
Three herbal remedies containing the infection-fighting…
-

-
Teasel
A traditional herb for healing bones and joints…
-

-
Barberry and Healthy Personal Boundaries
A thorny shrub for fighting infections and supporting…
December
-

-
The Evidence for Berberine
A yellow alkaloid found in traditional infection-fighting…
-

-
The Sensible Use of Caffeinated Herbs
Kola nuts, guarana, and yerba mate and other herbs…
-

-
The Health Benefits and Problems with Coffee
This popular caffeinated beverage can be beneficial…
October
-

-
Understanding Caffeine & Cellular Adaptation
Preserving the power of caffeine's buzz and the…
September
-

-
Horseradish
A pungent spice for aiding protein metabolism…
-

-
Banaba or Crepe Myrtle
A beautiful tree from Southeast Asia whose leaves…
August
-

-
Monkeyflowers
Flower essences to help see ourselves more clearly…
-

-
Mariposa Lilies
Strengthening the bond between mother and child…
-

-
The Noble Bay Leaf
A common kitchen herb for aiding digestion and…
-

-
Epimedium: Horny Goat Weed
A circulatory stimulant and kidney yang tonic…

